A complex unit for a complex disease: the HCM-Family Unit
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
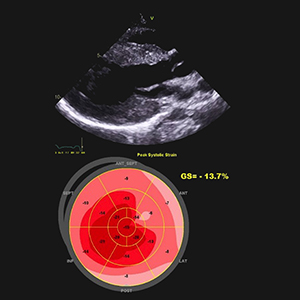
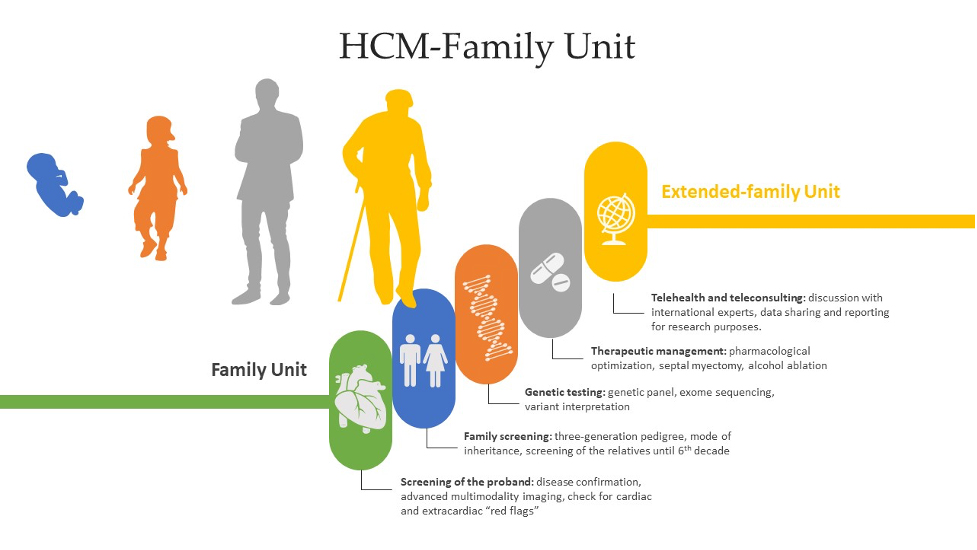
Hypertrophic cardiomyopathy (HCM) is a group of heterogeneous disorders that are most commonly passed on in a heritable manner. It is a relatively rare disease around the globe, but due to increased rates of consanguinity within the Kingdom of Saudi Arabia, we speculate a high incidence of undiagnosed cases. The aim of this paper is to elucidate a systematic approach in dealing with HCM patients and since HCM has variable presentation, we have summarized differentials for diagnosis and how different subtypes and genes can have an impact on the clinical picture, management and prognosis. Moreover, we propose a referral multi-disciplinary team HCM-Family Unit in Saudi Arabia and an integrated role in a network between King Faisal Hospital and Inherited and Rare Cardiovascular Disease Unit-Monaldi Hospital, Italy (among the 24 excellence centers of the European Reference Network (ERN) GUARD-Heart).
Graphical Abstract
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






