A complex unit for a complex disease: the HCM-Family Unit
Accepted: November 30, 2021
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
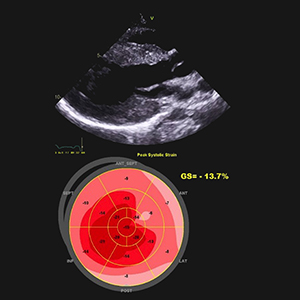
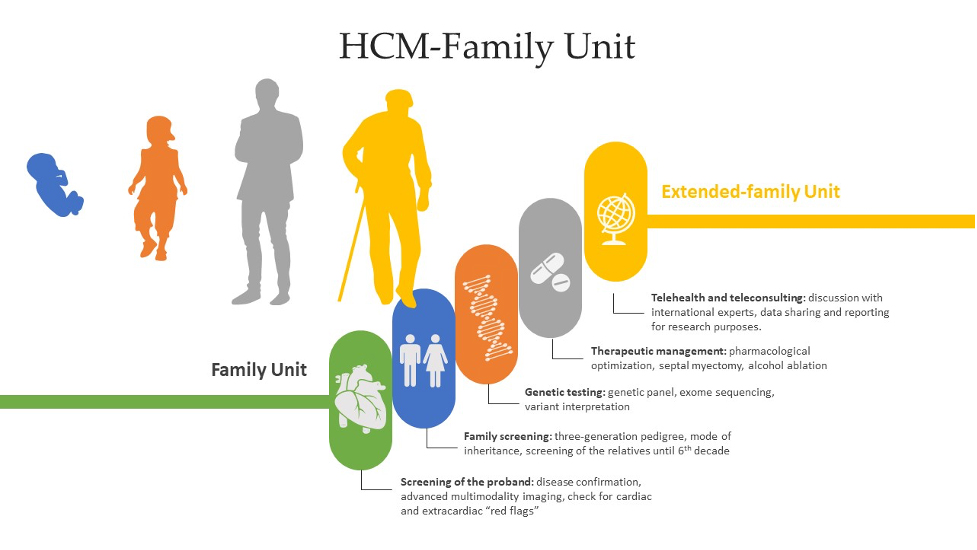
Hypertrophic cardiomyopathy (HCM) is a group of heterogeneous disorders that are most commonly passed on in a heritable manner. It is a relatively rare disease around the globe, but due to increased rates of consanguinity within the Kingdom of Saudi Arabia, we speculate a high incidence of undiagnosed cases. The aim of this paper is to elucidate a systematic approach in dealing with HCM patients and since HCM has variable presentation, we have summarized differentials for diagnosis and how different subtypes and genes can have an impact on the clinical picture, management and prognosis. Moreover, we propose a referral multi-disciplinary team HCM-Family Unit in Saudi Arabia and an integrated role in a network between King Faisal Hospital and Inherited and Rare Cardiovascular Disease Unit-Monaldi Hospital, Italy (among the 24 excellence centers of the European Reference Network (ERN) GUARD-Heart).
Graphical Abstract
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.
Similar Articles
- Angelica Cersosimo, Mauro Riccardi, Ludovica Amore, Giuliana Cimino, Gianmarco Arabia, Marco Metra, Enrico Vizzardi, Varicella zoster virus and cardiovascular diseases , Monaldi Archives for Chest Disease: Vol. 93 No. 2 (2023)
- Giorgio E. Polistina, Francesca Simioli, Pasquale Imitazione, Maurizia Lanza, Anna Annunziata, Giuseppe Fiorentino, Different presentation of pulmonary parenchymal disruption in COVID-19 pneumonia. Case series of Sub-Intensive Care Unit in Naples, Italy , Monaldi Archives for Chest Disease: Vol. 90 No. 4 (2020)
- C. Gurioli, G.L. Casoni, C. Gurioli, S. Tomassetti, M. Romagnoli, C. Ravaglia, V. Poletti, Endobronchial ultrasound in Dieulafoy's disease of the bronchus: an additional application of EBUS , Monaldi Archives for Chest Disease: Vol. 73 No. 4 (2010): Pulmonary series
- Joana Lourenço, Ana Paula Vaz, Rosa Anita Fernandes, Cristina Lopes, Ana Luísa Fernandes, Evaluation of patients’ satisfaction with domiciliary biological treatment in severe asthma: a Portuguese survey , Monaldi Archives for Chest Disease: Early Access
- Marta Lazzeri, Andrea Lanza, Raffaella Bellini, Angela Bellofiore, Simone Cecchetto, Alessia Colombo, Francesco D'Abrosca, Cesare Del Monaco, Giuseppe Gaudiello, Mara Paneroni, Emilia Privitera, Mariangela Retucci, Veronica Rossi, Martina Santambrogio, Maurizio Sommariva, Pamela Frigerio, Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR) , Monaldi Archives for Chest Disease: Vol. 90 No. 1 (2020)
- Vincenzo Russo, Anna Rago, Nunzia Laezza, Pierpaolo Di Micco, Laura Giannetti, Luigi Atripaldi, Antonio D'Onofrio, Paolo Golino, Gerardo Nigro, Edoxaban in elderly patient with morbid obesity and atrial fibrillation: the role of plasma levels evaluation for selecting the appropriate dose , Monaldi Archives for Chest Disease: Vol. 90 No. 1 (2020)
- Sanatkumar Bharamu Nyamagoud, A H M Viswanatha Swamy, Anchu S P, Sonia S Gaitonde, Jaison M Johnson, Vishwanath Hegadal, RETRACTED: Prescription analysis emphasizing on medication adherence of antibiotics for lower respiratory tract infection , Monaldi Archives for Chest Disease: Early Access
- Cristina Albrici, Jacopo Cefalo, Michele Mondoni, Alessia Moro, Manuela Bimbatti, Umberto Gianelli, Stefano Centanni, Left pleural effusion in a young woman with genital tuberculosis , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
- Caterina Oriana Aragona, Andrea Bianco, Roberto Caruso, Massimo Cerulli, Nicola Cosentino, Antonio Cittadini, Michele Gabriele, Mario Mallardo, Roberto Marini, Bruna Miserrafiti, Pietro Palermo, Alfonso Galati, Lipid-lowering therapy in patients with coronary heart disease: an Italian real-life survey. Results from the Survey on Risk FactOrs and CardiovascuLar secondary prEvention and drug strategieS (SOFOCLES) in Italy , Monaldi Archives for Chest Disease: Early Access
- Ana Filipa Amador, Catarina Martins da Costa, João da Silva Santos, Cláudia Camila Dias, Elisabete Martins, First-degree atrioventricular block in hypertrophic cardiomyopathy patients: an easy and worthy prognostic marker? , Monaldi Archives for Chest Disease: Early Access
<< < 7 8 9 10 11 12 13 14 15 16 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/monaldi.2021.2147
https://doi.org/10.4081/monaldi.2021.2147




