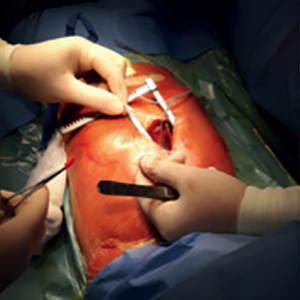
Subcutaneous finger cardioverter-defibrillator in low weight paediatric patients: a case series
Submitted: January 15, 2022
Accepted: June 20, 2022
Published: August 5, 2022
Accepted: June 20, 2022
Abstract Views: 992
PDF: 301
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Andrea Lanza, Maurizio Sommariva, Sara Mariani, Gabriela Ferreyra, Giuliana Enrica Stagni, Valeria Tombini, Angela Oppizzi, Catia Pontiggia, Andrea Bellone, Prolonged non-invasive respiratory supports in a COVID-19 patient with severe acute hypoxemic respiratory failure , Monaldi Archives for Chest Disease: Vol. 92 No. 1 (2022)
- Federico Raimondi, Caterina Conti, Luca Novelli, Francesco Tarantini, Giuseppe Ciaravino, Piermario Scuri, Aurelia Grosu, Daniela Chinaglia, Lorenzo S.C. Grazioli, Andrea Gianatti, Ferdinando L. Lorini, Michele Senni, Fabiano Di Marco, A 57-year-old man with rapidly progressive pulmonary hypertension , Monaldi Archives for Chest Disease: Vol. 92 No. 1 (2022)
- Sara Salim Ali, Imran Ahmed Qureshi, Ahmed Ayaz, Ainan Arshad, Awais Farhad, Bushra Jamil, Muhammad Rizwan Sohail, Etiology, clinical characteristics, and outcome of infective endocarditis: 10-year experience from a tertiary care center in Pakistan , Monaldi Archives for Chest Disease: Vol. 92 No. 4 (2022)
- Federico Raimondi, Stefano Centanni, Fabrizio Luppi, Stefano Aliberti, Francesco Blasi, Paola Rogliani, Claudio Micheletto, Marco Contoli, Alessandro Sanduzzi Zamparelli, Marialuisa Bocchino, Paolo Busatto, Luca Novelli, Simone Pappacena, Luca Malandrino, Giorgio Lorini, Greta Cairoli, Fabiano Di Marco, Respiratory rate-oxygenation index on the 3rd day is the best predictor of treatment failure in COVID-19 patients , Monaldi Archives for Chest Disease: Early Access
- Gian Francesco Mureddu, Stefano Nistri, Anna Maria Gori, Pompilio Faggiano, Biagio Fimiani, Antonio Maggi, Gianfranco Misuraca, Massimo Uguccioni, Luigi Tavazzi, Giovanni Battista Zito, Awareness and appropriateness of the management of preclinical heart failure in outpatient clinics in Italy: Insights from the VASTISSIMO study - EValuation of the AppropriateneSs of The preclInical phase (Stage A and Stage B) of Heart FaIlure Management in Outpatient Clinics in Italy , Monaldi Archives for Chest Disease: Vol. 89 No. 1 (2019)
- Michele Vitacca, Cinzia Lastoria, Monica Delmastro, Domenico Fiorenza, Pasquale De Cata, Barbara Fusar Poli, Sonia Gilè, Paola Prometti, Mara Paneroni, Cristina Bianchi, Elena Mandora, Roberta Porri, Claudio Fracchia, Use of inhaled devices during a hospital exacerbation of COPD: a summary of an interdisciplinary audit held at ICS Maugeri Pavia, Italy (March-June 2019) , Monaldi Archives for Chest Disease: Vol. 90 No. 1 (2020)
- Amarendra Kumar Shukla, Amrutha Peter, Jitendra Kishore Bhargava, Veerendra Arya, Manish Kumar Gupta, Nishtha Yadav, Pawan Tiwari, Sarcoidosis presenting as bilateral optic neuritis after ChAdOx1 nCoV-19 vaccination , Monaldi Archives for Chest Disease: Vol. 93 No. 1 (2023)
- Emanuele Stirpe, Floriana Bardaro, Johanna Köhl, Gluteal muscle metastases from malignant pleural mesothelioma: a case report , Monaldi Archives for Chest Disease: Early Access
- Sanchit Mohan, Rohit Kumar, Pranav Ish, Rajnish Kaushik, Tanmaya Talukdar, Neeraj Gupta, Nitesh Gupta, Diffuse alveolar hemorrhage: a retrospective study from a tertiary care center , Monaldi Archives for Chest Disease: Early Access
- Manuel Monti, Management of severe Falciparum malaria on a mission: A case report , Monaldi Archives for Chest Disease: Vol. 89 No. 1 (2019)
<< < 26 27 28 29 30 31 32 33 34 35 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/monaldi.2022.2203
https://doi.org/10.4081/monaldi.2022.2203





