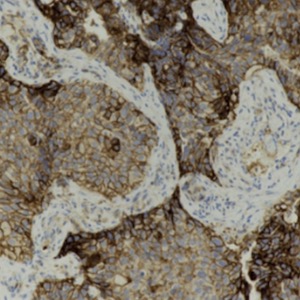
Complete response to pembrolizumab as a single agent in a patient with stage III NSCLC with high PD-L1 expression: a case report
Submitted: September 27, 2022
Accepted: November 14, 2022
Published: November 25, 2022
Accepted: November 14, 2022
Abstract Views: 1389
PDF: 334
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Giovanni Luca Botto, Carlo Piemontese, Giovanni Russo, Rhythm-control vs rate-control in the elderly: When to do it and which drug to prefer? , Monaldi Archives for Chest Disease: Vol. 88 No. 2 (2018)
- Concezione Tommasino, Cardiovascular risk assessment in the senior population undergoing anesthesia for non-cardiac surgery , Monaldi Archives for Chest Disease: Vol. 87 No. 2 (2017)
- Niccolò Marchionni, Francesco Orso, Cholesterol: until which age 'the lower the better'? , Monaldi Archives for Chest Disease: Vol. 84 No. 1-2 (2015): Cardiac series
- Sergio C. Conte, Giulia Spagnol, Marco Confalonieri, Beatrice Brizi, Deep sedation versus minimal sedation during endobronchial ultrasound transbronchial needle aspiration , Monaldi Archives for Chest Disease: Vol. 88 No. 3 (2018)
- Paolo Ruggeri, Federica Lo Bello, Francesco Nucera, Michele Gaeta, Francesco Monaco, Gaetano Caramori, Giuseppe Girbino, Hereditary hyperhomocysteinemia associated with nephrotic syndrome complicated by artery thrombosis and chronic thromboembolic pulmonary hypertension: A case report , Monaldi Archives for Chest Disease: Vol. 87 No. 3 (2017)
- H. Halilcolar, S. Yapicioglu, S. Bilaceroglu, Temporal Changes in Lung Cancer: A 10-year Study in a Chest Hospital , Monaldi Archives for Chest Disease: Vol. 69 No. 4 (2008): Pulmonary series
- C. Ortolani, F. Agostinis, S. Amoroso, R. Ariano, A. Barbato, M. Bassi, G. Cadario, P. Campi, F. Cardinale, G. Ciprandi, R. D’Anneo, M. Di Gioacchino, V. Di Rienzo, A. Fiocchi, M. Galimberti, E. Galli, M. Giovannini, C. Incorvaia, S. La Grutta, C. Lombardi, F. Marcucci, G. Marseglia, M. Minelli, A. Musarra, E. Nettis, E. Novembre, G. Pajno, G. Patriarca, F. Pezzuto, P. Piras, S. Pucci, A. Romano, C. Romano, O. Quercia, G. Scala, D. Schiavino, G. Sforza, M. Tosca, S. Tripodi, F. Frati, Practice parameters for sublingual immunotherapy , Monaldi Archives for Chest Disease: Vol. 65 No. 1 (2006): Pulmonary series
- Giampaolo Scorcu, Annarita Pilleri, Treatment of heart failure in the elderly: Which drugs are essential and which should be avoided , Monaldi Archives for Chest Disease: Vol. 88 No. 2 (2018)
- Fabrizio Corsini, Anna Scaglione, Maria Iacomino, Giuseppe Mascia, Saverio Melorio, Carmine Riccio, Salvatore Romano, Alfredo Vetrano, Silvana Celardo, Giancarlo Corsini, Carmelo Chieffo, Acute myocardial infarction in the elderly. A case-control study with a younger population and review of literature , Monaldi Archives for Chest Disease: Vol. 66 No. 1 (2006): Cardiac series
- Muhammad Ijaz, Muhammad Jaffar Khan, Jawad Khan, . Usama, Association of clinical characteristics of patients presenting with influenza like illness or severe acute respiratory illness with development of acute respiratory distress syndrome , Monaldi Archives for Chest Disease: Vol. 87 No. 1 (2017)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/monaldi.2022.2440
https://doi.org/10.4081/monaldi.2022.2440





