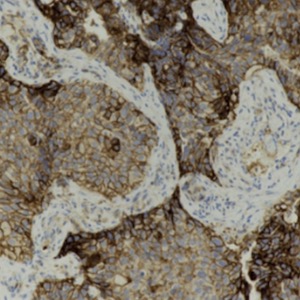
Complete response to pembrolizumab as a single agent in a patient with stage III NSCLC with high PD-L1 expression: a case report
Submitted: September 27, 2022
Accepted: November 14, 2022
Published: November 25, 2022
Accepted: November 14, 2022
Abstract Views: 1190
PDF: 303
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Nousheen Iqbal, Iffat Khanum, Muhammad Ali Ibrahim Kazi, Syeda Urooj Riaz, Uzzam Ahmed Khawaja, Safia Awan, Muhammad Irfan, Ali Bin Sarwar Zubairi, Javaid Ahmed Khan, Post-COVID-19 sequelae of the respiratory system. A single-centre experience reporting the compromise of the airway, alveolar and vascular components , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
- Angelica Cersosimo, Mauro Riccardi, Ludovica Amore, Giuliana Cimino, Gianmarco Arabia, Marco Metra, Enrico Vizzardi, Varicella zoster virus and cardiovascular diseases , Monaldi Archives for Chest Disease: Vol. 93 No. 2 (2023)
- Fulvio Cacciapuoti, Ilaria Caso, Mario Crisci, Fabio Minicucci, Federico Cacciapuoti, An unexpected cause of chest pain, dyspnea and palpitations in a young patient during a post-COVID syndrome , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
- Nishant Kumar Chauhan, Ashish Agarwal, Naveen Dutt, Taruna Yadav, Rishabh Kochar, Pulmonary embolism and gastric bleed with disseminated mucormycosis - treading dangerous waters , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
- Nabila Kanwal, Humza Thobani, Ainan Arshad, Priya Ashok Kumar, Fatima Amjad, Safia Awan, Muhammad Irfan, Factors predicting mortality among patients with COVID-19 associated hospital acquired pneumonia: insights from a tertiary care center , Monaldi Archives for Chest Disease: Vol. 93 No. 4 (2023)
- Gennaro Ratti, Cinzia Monda, Federica Ratti, Marco Golino, Ludovica Fulgione, Cosimo Fulgione, Mario Mallardo, Paolo Tammaro, Long-term dual antiplatelet therapy and nuisance bleeding: impact on quality of life , Monaldi Archives for Chest Disease: Vol. 93 No. 4 (2023)
- Martina Vigorè, Nicolo Granata, Giovanna Callegari, Raffaella Vaninetti, Simona Conti, Roberto Maestri, Giancarlo Piaggi, Gioele Cremonese, Antonia Pierobon, Frailty and rehabilitation outcome in older patients with cardiorespiratory disease: preliminary multidimensional data , Monaldi Archives for Chest Disease: Vol. 93 No. 4 (2023)
- Davide Papi, Giulia Montigiani, Luca Bucciardini, How the work of respiratory physiotherapists changes the tracheostomy management and decannulation in a NICU department: an Italian experience , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
- Georgi Yankov, Magdalena Alexieva, Nikolay Yanev, Evgeni Mekov, Two cases with postintubation tracheal stenosis after COVID-19 pneumonia , Monaldi Archives for Chest Disease: Vol. 93 No. 4 (2023)
- Naoto Ishimaru, Yohei Kanzawa, Takahiro Nakajima, Kayoko Okamura, Eiichiro Sando, Isao Ito, Saori Kinami , Hisashi Ohnishi, Specific antibody deficiency to pneumococcal polysaccharide in a young adult with recurrent respiratory infections: a case report , Monaldi Archives for Chest Disease: Vol. 93 No. 3 (2023)
<< < 36 37 38 39 40 41 42 43 44 45 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/monaldi.2022.2440
https://doi.org/10.4081/monaldi.2022.2440





